Examples of pure substances include iron steel and water. Pure substances exhibit very well-defined physical properties or properties that are not connected with the substances ability to combine with different substances.

Re Post Element Compound And Mixture Tyas Physics Compounds And Mixtures Elements Compounds And Mixtures School Science Experiments
This section aims at reviewing and evaluating newly acquired knowle.

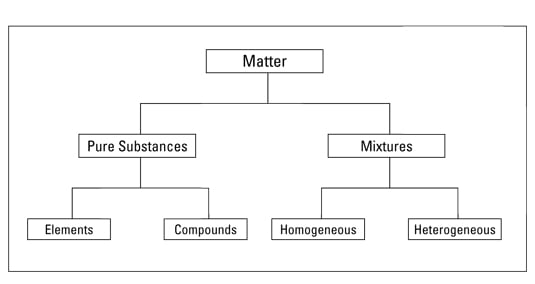
Pure substance vs mixture examples. By their chemical composition pure substances get divided into two types elements and compounds. You then end up with pure sand. Therefore the properties are uniform throughout the sample.
You can do this by adding water dissolving the salt and then filtering the mixture. For example suppose you have a mixture of salt and sand and you want to purify the sand by removing the salt. A pure substance cannot be physically separated into other substances because all of the particles are the same.
In chemistry the pure substance is a very simple concept to grasp. Its a substance made of only one type of atom or only one type of molecule a cluster of atoms bonded together. Solid and liquid such as sand and water.
Mixtures can be classified into two types viz. Some of the examples of pure substances are gold platinum copper nitrogen gas oxygen gas pure water etc. For example shops sell cartons labelled as pure orange juice.
If an element or compound exists in two states simultaneously it can be a pure substance and a mixture at the same time. Gas and gas like nitrogen and oxygen in the atmosphere. As a mixture the ice can be separated from the water with physical means such as scooping out the ice fragments.
The differences between pure substances and mixture are given below. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. A pure substance.
1 Pure substance The substances that contain only one type of particle and they are free from any mixture are known as pure substances. In this article we are going to focus on pure substances. Some common examples of mixtures include.
Examples of Pure Substances Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Which is an example of a container filled with a pure substance. A mixture on the other hand is the physical combination of two or more substances.
Gas and liquid such as water. Pure substances are homogenous. In contrast mixtures contain two or more substances so they can be separated.
Differences Between Pure Substances and Mixtures. Whereas that of the mixture is sugar syrup alcohol sand oil etc. Pure substance cannot be separated into two or more substances by any mechanical or physical method.
Heres a video from the Evaluate section in the Pure Substances and Mixtures 5E Lesson. Pure substances and mixtures The meaning of pure The word pure is used in chemistry in a different way from its everyday meaning. Mixtures show the properties of the pure substances in it.
For example pure water with pure crushed ice in it is still a pure substance but it is also a mixture of two states of the pure substance. Pure substances are further divided into elements and compounds. Gold silver iron and aluminium are pure substances to name a few.
Examples of pure substances include chemical elements and compounds. A solution like water and oil. Oxygen water and iron are examples of pure substances.
A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together Distinguishing between pure substances and. Examples of pure substances include oxygen water and iron. Although water is a pure substance if you put sand into a glass of water it would turn into a mixture.
Mixtures can be either homogeneous or heterogeneous. Alloys and other solutions may also be considered pure if they have a constant composition. Each of the components of a mixture can be separated from one another.
The physical and the chemical properties such and melting and the boiling points of the components of the pure substance remain the same whereas they vary differently in a mixture. You can always separate the sand from water by filtering it. A pure substance is made up of same kinds of molecules elements and compounds are the basic examples of such matter whereas mixture is made up of two different kinds of molecules homogeneous mixtures and heterogeneous mixtures are the major types of mixtures.
Heterogeneous and homogeneous mixtures. Pure Substances vs Mixtures DRAFT. The combination of two or more pure substances is called a mixture.
Pure Substances vs Mixtures DRAFT. Pure substances have fixed physical properties such as melting and boiling point. Air is a homogeneous mixture that is often considered to be a pure substance.
Pure Substances And Mixtures Bioprofe
How To Distinguish Pure Substances And Mixtures Dummies

What Are The Types Of Pure Substances Compounds Elements Videos
/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)